I have recently come across the total synthesis of (–)-auxofuran by Boukouvalas and Loach (from Université Laval in Québec) and it is really the kind of synthesis I like: short, efficient, well thought.1 The central feature, namely the construction of a 3,4-disubstituted furan, is particularly interesting.
Furans are notoriously difficult to functionalise on other positions than 2 and 5, whether you try a classic SEAr or a directed lithiation. Here the authors got around the synthetic difficulty by a de novo construction of the furan core from a disubstituted alkyne.
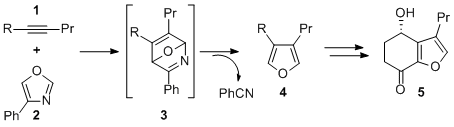 I like the way a pretty standard molecule such as 4-phenyloxazole can be used as a synthetic equivalent of “dimethine ether” or as a super-carbonyl ylide.
I like the way a pretty standard molecule such as 4-phenyloxazole can be used as a synthetic equivalent of “dimethine ether” or as a super-carbonyl ylide.
 This is all fine and dandy, but I wondered how accessible 4-phenyloxazole was. I could not find it in the catalogues of common suppliers, so I guess you would have to make it yourself.
This is all fine and dandy, but I wondered how accessible 4-phenyloxazole was. I could not find it in the catalogues of common suppliers, so I guess you would have to make it yourself.
I guess I can already rule out the van Leusen oxazole synthesis since this reaction affords 5-substituted oxazoles.
 I was thinking of using oxazole directly and start from there, but with a cost of £229 for only 10 g of starting material (price found in the Sigma-Aldrich catalogue), I turned to a less risky plan. It seems that the strategy of Rickborn et al.2 is somehow the most reasonable. One step from cheap commercially available starting materials (2-bromoacetophenone and ammonium formate are not particularly expensive stuff…), the yield is the only drawback.
I was thinking of using oxazole directly and start from there, but with a cost of £229 for only 10 g of starting material (price found in the Sigma-Aldrich catalogue), I turned to a less risky plan. It seems that the strategy of Rickborn et al.2 is somehow the most reasonable. One step from cheap commercially available starting materials (2-bromoacetophenone and ammonium formate are not particularly expensive stuff…), the yield is the only drawback.
 Whilst fathoming the literature for interesting strategies, I came across some very interesting photochemical rearrangements from 4-phenylisoxazole3 (94% yield in 5 min!) and 2-phenyloxazole4 leading to 4-phenyloxazole. Agreed, it would lengthen the synthesis but, to me at least, it seems so much more interesting (mechanism-wise). On the same topic, the photo-mediated addition of 1,3-dioxole to benzonitrile seems to give an original access to 4-phenyloxazole, albeit in low yield.5
Whilst fathoming the literature for interesting strategies, I came across some very interesting photochemical rearrangements from 4-phenylisoxazole3 (94% yield in 5 min!) and 2-phenyloxazole4 leading to 4-phenyloxazole. Agreed, it would lengthen the synthesis but, to me at least, it seems so much more interesting (mechanism-wise). On the same topic, the photo-mediated addition of 1,3-dioxole to benzonitrile seems to give an original access to 4-phenyloxazole, albeit in low yield.5
One last interesting way to synthesise 4-phenyloxazole I found was the Lewis acid-mediated rearrangement of 3-phenyl-2H-azirine-2-carbaldehyde.6 Oddly enough, this compound rearranges into two different isomers depending on the type of species it is treated with. In presence of transition metal complexes (of Rh and Ru, here TM) 3-phenylisoxazole is produced while main group Lewis acids (BF3.OEt2, AlCl3, InCl3, here LA) tend to favor 4-phenyloxazole.
The second part or this article, a focus on the chemistry of azirines is to come for next time.
References:
1. Org. Lett. 2013, 15, 4912-4915. link
2. J. Org. Chem. 1990, 55, 929-935. link
3. J. Heterocycl. Chem. 2005, 42, 273-281. link
4. J. Chem. Soc., Perkin Trans. 1 1977, 239-247. link
5. Tetrahedron 1987, 43, 5781-5790. link
6. J. Heterocycl. Chem. 2008, 45, 311-317. link

